Draw The Major Elimination Product Formed In The Reaction
Draw The Major Elimination Product Formed In The Reaction - Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Web the major product of an elimination reaction tends to be the more substituted alkene. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web draw the major elimination product formed in the following reaction. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. There are 3 steps to solve this one. Web in many cases one major product will be formed, the most stable alkene.
draw the major elimination product formed in the reaction share4uhoshiro
Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. There are 3 steps to solve this one. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. Web if two or more structurally distinct groups of adjacent hydrogens are present in.
[Solved] draw the major product formed. Draw the major elimination product... Course Hero
In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web the major product of an elimination reaction tends to be the more substituted alkene. This is because the transition state.
Solved Draw the major elimination product formed in the
Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. There are 3 steps to solve this one. Web if two or more.
draw the major elimination product formed in the reaction redandyellowvans
This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric.
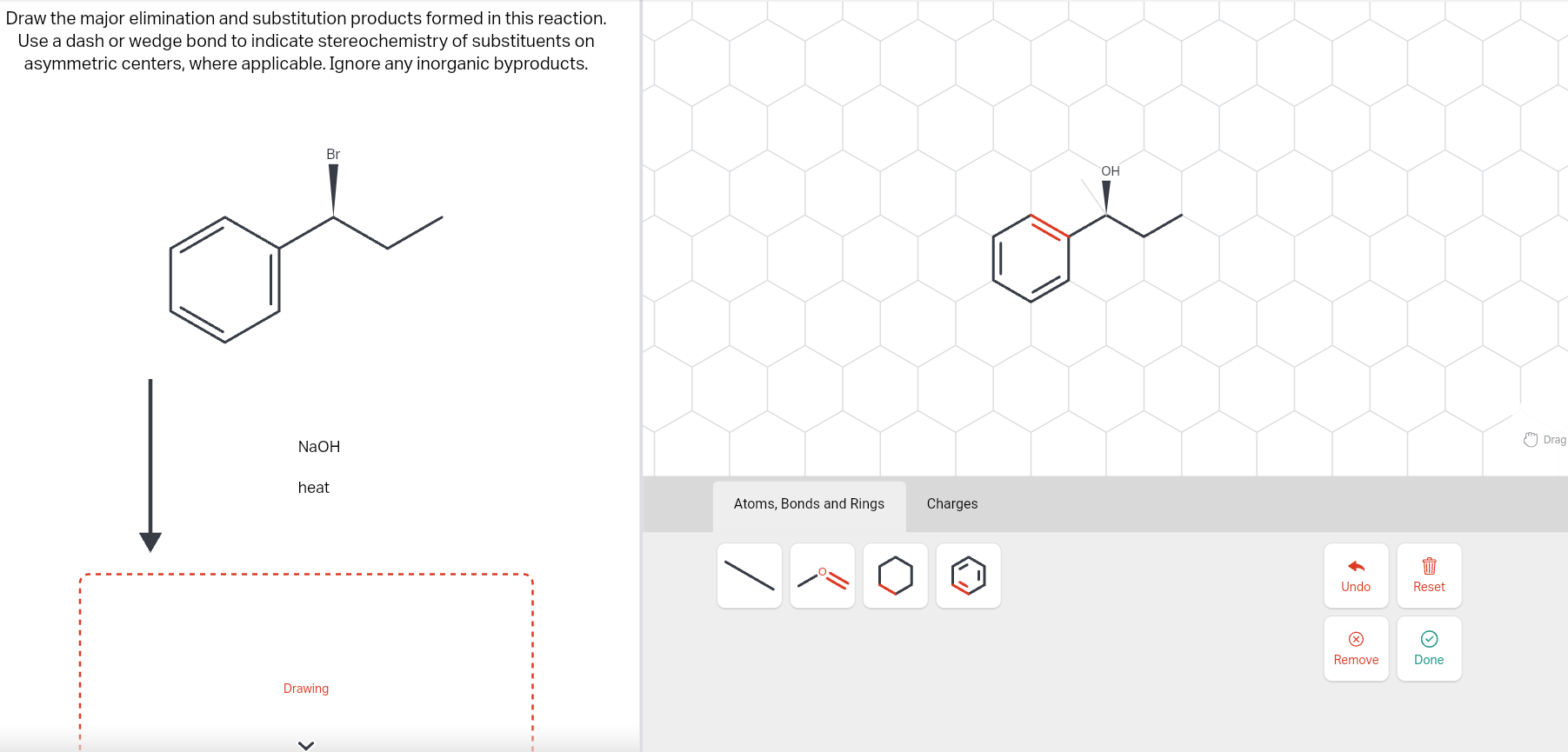
Solved Draw the major elimination and substitution products
This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. Web in many cases one major product will be formed, the most stable.
Solved Draw the major elimination product formed in the
This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Web the major product of an elimination reaction.
Solved Draw the major elimination and substitution products
Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Web the major product of an elimination reaction tends to be the more substituted alkene. Web in many cases one major product will be formed, the most stable alkene. There are 3.
Solved Draw the major elimination and substitution products
Web draw the major elimination product formed in the following reaction. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. There are 3 steps to solve this one. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to.
Draw the major elimination product formed in the reaction. Select Draw Rings More CH3OK+ DMSO
There are 3 steps to solve this one. Web in many cases one major product will be formed, the most stable alkene. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Alkyl halides undergo elimination via two common mechanisms, known as.
Solved Draw the major elimination and substitution products
In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Web the major product of an elimination reaction tends to be the.
Web the major product of an elimination reaction tends to be the more substituted alkene. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. There are 3 steps to solve this one. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate. Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then. Web in many cases one major product will be formed, the most stable alkene. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination. Web draw the major elimination product formed in the following reaction.
This Is Because The Transition State Leading To The More Substituted Alkene Is Lower In Energy And Therefore Will Proceed At A Higher Rate.
There are 3 steps to solve this one. Zaitsev’s rule is an empirical rule used to predict the major products of elimination reactions. Web draw the major elimination product formed in the following reaction. Web to find the products in a specific case, locate the hydrogen atoms on each carbon next to the leaving group, and then.
Web In Many Cases One Major Product Will Be Formed, The Most Stable Alkene.
Alkyl halides undergo elimination via two common mechanisms, known as e2 and e1, which show some similarities to s n 2 and s. In terms of regiochemistry, zaitsev’s rule states that when more than one product can be formed, the more substituted alkene is the major product. Web the major product of an elimination reaction tends to be the more substituted alkene. Web if two or more structurally distinct groups of adjacent hydrogens are present in a given reactant, then multiple constitutionally isomeric alkenes may be formed by an elimination.