Two Point Form Of Arrhenius Equation
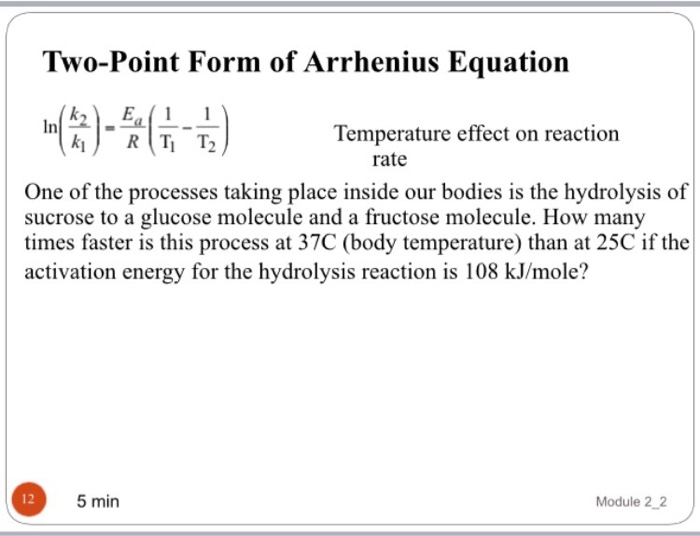
Two Point Form Of Arrhenius Equation - The arrhenius equation can determine an unknown rate constant or temperature. Web the arrhenius equation, k = ae − ea / rt. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system.
Two point arrhenius equation, Easy derivation, 3 application Chemistry Notes
Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. The arrhenius equation can determine an unknown rate constant or temperature. Web the arrhenius equation, k = ae − ea / rt.
Chapter 15 Chemical ppt download
Web the arrhenius equation, k = ae − ea / rt. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. The arrhenius equation can determine an unknown rate constant or temperature.
TwoPoint Form for Arrhenius Equation YouTube
Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. The arrhenius equation can determine an unknown rate constant or temperature. Web the arrhenius equation, k = ae − ea / rt.
Solved Complete the twopoint form of the Arrhenius
The arrhenius equation can determine an unknown rate constant or temperature. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. Web the arrhenius equation, k = ae − ea / rt.
Two point arrhenius equation, Easy derivation, 3 application Chemistry Notes
The arrhenius equation can determine an unknown rate constant or temperature. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. Web the arrhenius equation, k = ae − ea / rt.
Solved TwoPoint Form Of Arrhenius Equation In N2. Temper...
Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. Web the arrhenius equation, k = ae − ea / rt. The arrhenius equation can determine an unknown rate constant or temperature.
Two Point Form Of Arrhenius Equation Free Printable Form
Web the arrhenius equation, k = ae − ea / rt. The arrhenius equation can determine an unknown rate constant or temperature. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system.
PPT The Arrhenius Equation PowerPoint Presentation, free download ID1115664
Web the arrhenius equation, k = ae − ea / rt. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. The arrhenius equation can determine an unknown rate constant or temperature.
The other forms of the Arrhenius Equation YouTube
The arrhenius equation can determine an unknown rate constant or temperature. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system. Web the arrhenius equation, k = ae − ea / rt.
Chapter 13 Chemical ppt download
The arrhenius equation can determine an unknown rate constant or temperature. Web the arrhenius equation, k = ae − ea / rt. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system.
The arrhenius equation can determine an unknown rate constant or temperature. Web the arrhenius equation, k = ae − ea / rt. Web in order to calculate the activation energy we need an equation that relates the rate constant of a reaction with the temperature (energy) of the system.
Web In Order To Calculate The Activation Energy We Need An Equation That Relates The Rate Constant Of A Reaction With The Temperature (Energy) Of The System.
The arrhenius equation can determine an unknown rate constant or temperature. Web the arrhenius equation, k = ae − ea / rt.